how to find percent ionization
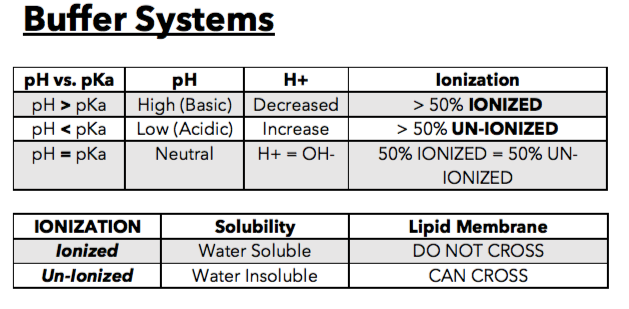
Ok, now on to the most important part of acid bases. This is essentially a concept based question that I have multiple times. Essentially, you're gonna want to know the chart above. Follow with me.
if pH is greater than pKa, this means the following:
- the solution is very basic (high pH means it is a stronger base)
- therefore, there will be low H+ (since this is the acid portion, its obviously gonna be low if there is more base aka OH-)
- resulting in more ionization (greater than 50%)
- Bottomline: More Base = More Ionization
if pH is less than pKa, this means the following:
- the solution is very acid (low pH means it is a stronger acid)
- therefore, there will be high H+ (since this is the acid portion, its obviously gonna be higher if there is more acid than base aka OH-)
- resulting in less ionization (greater than 50%), or more commonly referred to as un-ionzation
- Bottomline: More Acid= Less Ionization
Last but not least, if ph= pKa, then its a neutral solution and everything is equal.
This is a crucial concept to know because drugs have certain levels of ionization that allow them to cross the lipid membrane and essentially be able to become therapeutically effective.
If a drug can't cross, it won't work as intended.
An example you may see on the naplex is like this.
Drug A has a pH of 3 and pKa of 6. Drug B has a pH of 8 and pKa of 4. Drug C has a pH of 5 and pKa of 5. Drug D has a pH of 4 and a pKa of 5. Drug E is neutral.
A patient requires a drug to be highly soluble in order to treat a certain condition. Which of the following drugs is the best solution of this patient?
- Drug A
- Drug B
- Drug C
- Drug D
- Drug E
Solution.
- Drug A — More than 50% unionization and water insoluble, can cross
- Drug B — More than 50% ionization and water soluble, cannot cross
- Drug C — 50% ionized + 50% unionized, neutral
- Drug D — More than 50% unionization and water insoluble, can cross
- Drug E — Neutral
So if you can guess right now you're thinking there are two correct answers, right? That is true, welcome to the NAPLEX. Get used to it. So how do we choose? Simple, the question asks for the "best solution". In this case, Drug A has a greater difference between pH and pKa than Drug D, therefore, Drug A is the best solution. Why? Think of ionization as something that adds bulk to a drug. If it is bulky, it won't be able to cross/fit through the membrane. Drug A is the best solution because the greater the difference between pH and pKa the larger the size difference. We can infer that Drug A is smaller than Drug D simply due to a greater difference between pH and pKa. So it's the best solution
Didn't think it would get that detailed did you? Well, to be honest, it really wasn't a hard question and you could still answer it effectively just by looking at my review. The key is that the questions will be simple and straightforward, but abstract and unique. They want you to think outside the box and make clear and confident decisions. Remember, being a pharmacist is not about how well you can memorize things or how smart you are (to be honest). It is really whether or not you are confident enough in yourself, your education, and training to allow yourself to answer these questions.
Thank You For Reading!
Did you find this post helpful? Perhaps even insightful or game changing? If so, please make sure to support my blog. As a small content curator, I am always looking for ways to provide new content.
Support Minimalist / Pharmacist
Patreon | Paypal | Square
Business
Legal | Contact: minimalistpharmacist@gmail.com
Social Media
Instagram | Facebook | Twitter | Tumblr| Google+ | Pinterest | Reddit
Amazon Favorites
Kindle Unlimited | Amazon Fresh | Amazon Music | Amazon Prime | Audible
Prime Student | Fire TV 4K | Amazon Home | Amazon Phones | Car Alexa
Personal Favorites
Spotify | Essential | Bambino | Lunar Tempo 2 | Oblivion | NordVPN
Running Gear
Nike Flex Running Shoes | Nike Element Running Top | Nike Essential Running Pants
Favorite Hamilton Watches
Dress Watch | Field Watch | Chronograph Watch
how to find percent ionization
Source: https://medium.com/minimalist-pharmacist/everything-you-need-to-know-about-ph-pka-91aeb6d61709
Posted by: andrewsbarl1983.blogspot.com

0 Response to "how to find percent ionization"
Post a Comment